PPI in HSC R&D Division
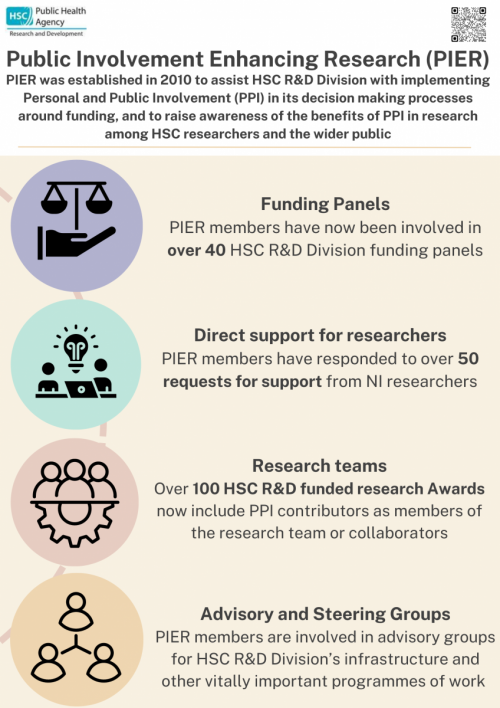
- As part of the Strategy for PPI in HSC Research, HSC R&D Division established a group of Personal and Public Involvement (PPI) representatives who support it in its activities. The name of this group is PIER (NI). PIER stands for Public Involvement Enhancing Research.
- The purpose of PIER (NI) is to help HSC R&D Division with implementing PPI in its decision-making processes and to raise awareness of the benefits of PPI in research among HSC researchers and the wider public. It is not intended for PIER members to represent any group of patients or organisation - they lend their own individual perspective as a member of the general public or a patient, service user or carer with experience of a particular illness, condition or service.
Get to know our current PIER members
Please click here to read the biographies of our current members.
You can also read more about our PIER member Noel Wilson and he became involved in research or listen to this presentation by Laura Collins on her reflections and considerations of PPI as a PIER Member (or read the transcript of this recording).
Interested in working with PIER?
If you are a researcher and you would like to work with PIER, please complete and submit the 'Request for Support' form.
We ask that anyone who engages with PIER gives careful consideration to the UK Standards for Public Involvement which have been designed to improve the quality and consistency of public involvement in research. You must ensure good communication and commit to providing feedback on completion of your project on how PIER NI member(s) involvement benefited and/or influenced your project.
If you have already worked with a member(s) of PIER and would like to provide your feedback, please complete the 'Researcher Feedback' form.
Interested in becoming a member of PIER (NI)?
Membership of PIER (NI) is voluntary and new members are welcomed to join on an on-going basis. If you would like to find out more please read the description of PPI role and complete and submit the 'Expression of Interest' form.
Already a member?
We also have an online form for PIER members to provide their feedback via the 'Member feedback' form.
Some members of PIER tell their story...

Researcher feedback on working with PIER members
“Comments from the PIER member were extremely helpful and informed our approach to ethical amendments to improve patient recruitment”
“Comments from PIER gave me really helpful feedback on participant questionnaire. The rationale made sense and improved upon the study design”
“Suggestions from the PIER member influenced changes we made to patient recruitment strategies (e.g. the phrasing used by pharmacists when first introducing the study, changes to the age limit) and wording changes to the patient study information sheets. Initial feedback from pharmacists has indicated that changing their language when approaching patients as recommended led to an increase in the number of patients who were recruited into the study”.
Page last updated 20.10.25

